دكسترين: الفرق بين النسختين
بدأ ترجمة من https://en.wikipedia.org/w/index.php?title=Dextrin&oldid=612948572 |
(لا فرق)
|
نسخة 19:19، 24 أكتوبر 2014
هذه مقالة أو قسم، تخضع لتحريرٍ مُكثَّفٍ في الفترة الحالية لفترةٍ قصيرةٍ. لتفادي تضارب التحرير؛ يُرجى عدم تعديل الصفحة في أثناء وجود هذه الرسالة. أُجري آخر تعديل على الصفحة في 19:19، 24 أكتوبر 2014 (UTC) ( منذ 9 سنين) – . فضلًا أزل هذا القالب لو لم تكن هنالك تعديلات على المقالة في آخر 24 ساعة. إذا كنت المحرر الذي أضاف هذا القالب، فضلًا تأكد من إزالته واستبداله بقالب {{تطوير مقالة}} بين جلسات التحرير. |
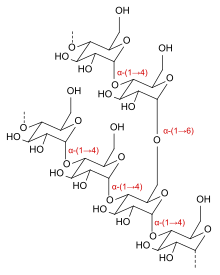
الدكسترينات هي مجموعة من الكربوهيدرات منخفضة الوزن الجزيئي تنتج بواسطة التحليل المائي للنشا[1] أو الجليكوجين.[2] الدكسترينات هي خليط من وحدات الجلوكوز المبلمرة المتصلة بالرابطة الجليكوسيدية α-(1→4) أو α-(1→6).
| دكسترين | |
|---|---|
| المعرفات | |
| رقم CAS | 9004-53-9 |
| بوب كيم (PubChem) | 62698 |
| الخواص | |
| الصيغة الجزيئية | (C6H10O5)n |
| الكتلة المولية | variable |
| المظهر | white or yellow powder |
| في حال عدم ورود غير ذلك فإن البيانات الواردة أعلاه معطاة بالحالة القياسية (عند 25 °س و 100 كيلوباسكال) | |
| تعديل مصدري - تعديل | |
يمكن انتاج الدكسترينات من النشا باستخدام إنزيم مثل الأميليز، وذلك كما يحدث أثناء الهضم في جسم الانسان وأثناء صناعة المولت والتسكير.[3] أو باستخدام الحرارة الجافة تحت ظروف حامضية (التحلل الحراري أو التحميص).
The latter process is used industrially, and also occurs on the surface of bread during the baking process, contributing to flavor, color, and crispness. Dextrins produced by heat are also known as pyrodextrins. During roasting under acid condition the starch hydrolyses and short chained starch parts partially rebranch with α-(1,6) bonds to the degraded starch molecule.[4] See also Maillard Reaction.
Dextrins are white, yellow, or brown powders that are partially or fully water-soluble, yielding optically active solutions of low viscosity. Most can be detected with iodine solution, giving a red coloration; one distinguishes erythrodextrin (dextrin that colours red) and achrodextrin (giving no colour).
White and yellow dextrins from starch roasted with little or no acid is called British gum.
الاستخدامات
Yellow dextrins are used as water-soluble glues [5] in remoistable envelope adhesives and paper tubes, in the mining industry as additives in froth flotation, in the foundry industry as green strength additives in sand casting, as printing thickener for batik resist dyeing, and as binders in gouache paint and also in the leather industry.
White dextrins are used as:
- a crispness enhancer for food processing, in food batters, coatings, and glazes, (E number 1400)
- a textile finishing and coating agent to increase weight and stiffness of textile fabrics
- a thickening and binding agent in pharmaceuticals and paper coatings.
As pyrotechnic binder and fuel, they are added to fireworks and sparklers, allowing them to solidify as pellets or "stars."
Due to the rebranching, dextrins are less digestible; indigestible dextrin are developed as soluble stand alone fiber supplements and for adding to processed food products.[6]
Other dextrin types
- Maltodextrin
Maltodextrin is a shortchain starch sugar used as a food additive. It is produced also by enzymatic hydrolysis from gelled starch and is usually found as a creamy-white hygroscopic spraydried powder. Maltodextrin is easily digestible, being absorbed as rapidly as glucose, and might either be moderately sweet or have hardly any flavor at all.
- Cyclodextrin
The cyclical dextrins are known as cyclodextrins. They are formed by enzymatic degradation of starch by certain bacteria, for example, Bacillus macerans. Cyclodextrins have toroidal structures formed by 6-8 glucose residues.
- Amylodextrin is a linear dextrin or short chained amylose (DP 20-30) that can be produced by enzymatic hydrolysis of the alpha-1,6 glycosidic bonds or debranching amylopectin. Amylodextrin colors blue with iodine.
- (Beta) Limit dextrin is the remaining polymer produced by enzymatic hydrolysis of amylopectin with beta amylase, which cannot hydrolyse the alpha-1,6 bonds at branch points.
- (Alpha) Limit dextrin is a short chained branched amylopectin remain, produced by hydrolysis of amylopectin with alpha amylase.
- Highly branched cyclic dextrin is a dextrin produced from enzymatic breaking of the amylopectin in clusters and using branching enzyme to form large cyclic chains.[7]
انظر أيضا
- Brewing
- Cellodextrin, breakdown of cellulose
- Dextrose equivalent
- Icodextrin
- Modified starch
- Starch gelatinization
References
- ^ An Introduction to the chemistry of plants - Vol II: Metabolic processes, P. Haas and T. G. Hill, London (Longmans, Green & Co.), 1913; pages 123-127
- ^ Salway, JG. Medical Biochemistry at a Glance. Second Edition. Malden, MA (Blackwell Publishing), 2006; page 66
- ^ Michael Lewis, Tom W. Young (2002), "Brewing", Kluwer Academic, ISBN 0-306-47274-0.
- ^ Alistair M. Stephen, Glyn O. Phillips, Peter A. Williams (2006), "Food polysaccharides and their applications 2nd edition", p 92-99, CRC Press, Taylor & Francis Group, ISBN 0-8247-5922-2
- ^ Jack Augustus Radley (1976). "Industrial uses of starch and its derivatives", Applied Science Publishers Ltd, ISBN 0-85334-691-7.
- ^ http://www.webmd.com/diet/fiber-health-benefits-11/compare-dietary-fibers
- ^ T. Hiroki, K. Iwao, T. Noboru,S. Yuji, Y. Mikio, Journal: Seibutsu Kogakkaishi, Vol:84; No:2; Page: 61-66 (2006), Industrial Production of Branching Enzyme and Its Application to Production of Highly Branched Cyclic Dextrin (Cluster Dextrin)[1]
External links
| ابحث عن دكسترين في ويكاموس. |
- Dextrins في المكتبة الوطنية الأمريكية للطب نظام فهرسة المواضيع الطبية (MeSH).
